- Solutions
- Clinical Registry
- Clinical Registry platform
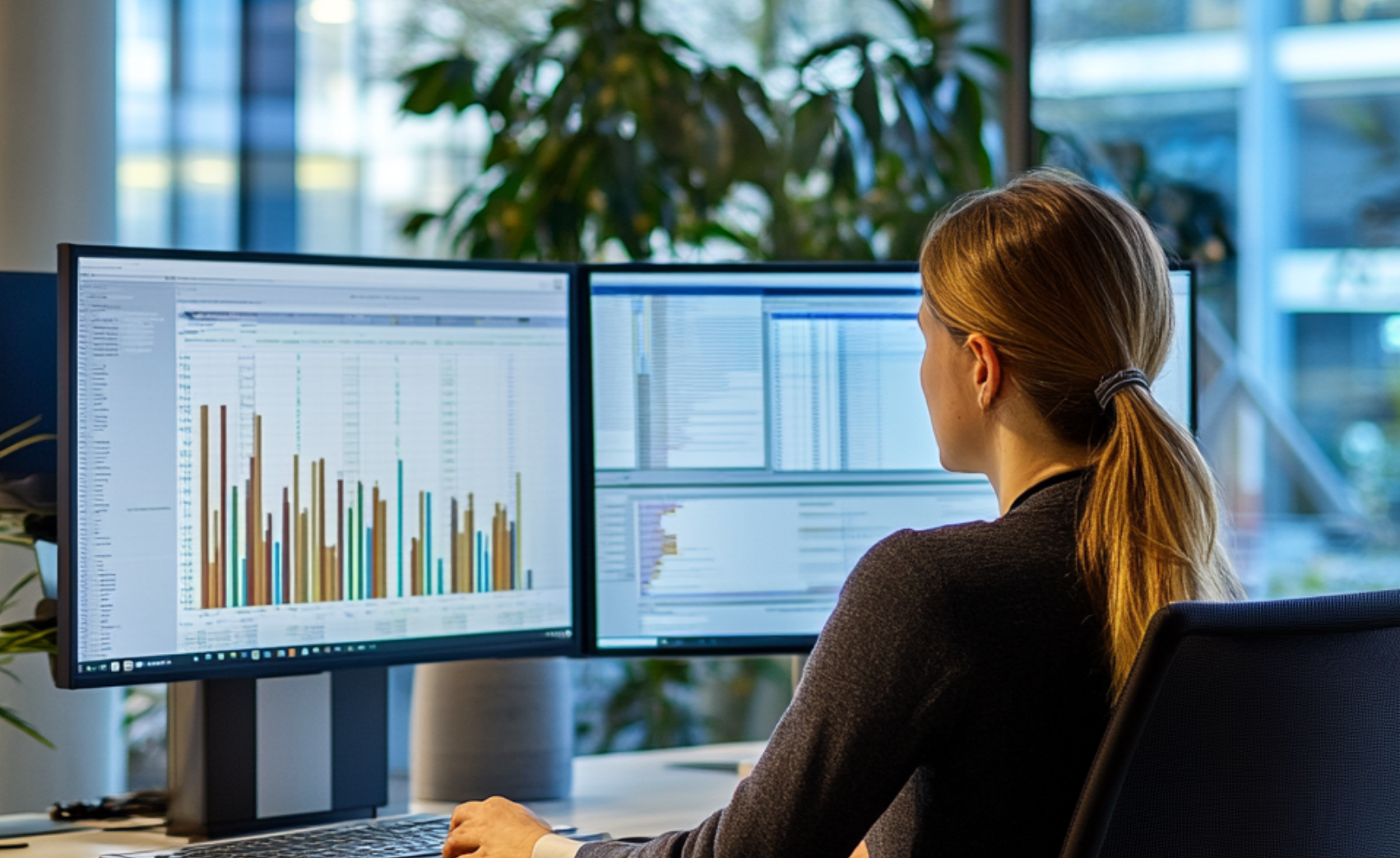
Clinical Registry Platform: Connect Today, Analyse Tomorrow.
Our custom-built Clinical Registry platform makes it easy for healthcare providers worldwide to collect, process, and analyse their outcomes data, deploying those findings towards better healthcare.
.jpg?width=1920&height=1280&name=iStock-1157269663%20(1).jpg)



Collect, process and report
Set up a clinical registry today, and let us take care of all the steps.
Report
Our dashboard allows for customisable views and insights into the overall registry performance nearly real-time, making it easy to identify outliers and best practices within specific therapeutic areas. The platform allows for sharing information with third parties and for connecting directly with peers to learn and improve.
.jpg?width=1920&height=1280&name=iStock-1157269663%20(1).jpg)
Pick and choose the features you require for your clinical registry.

DataEntry

DataConnect
Distribution

Automated data collection

Codman
Self Service Portal
Count on the excellent support of our Service Desk
Our Clinical Registry Platform comes with a service bundle that guarantees optimal support from our experts at the Clinical Registry Service Desk to help you get the most out of your platform.

Frequently Asked Questions
What is a clinical registry?
A clinical registry includes collection, storage and analysis of health data related to patients with (a combination of) specific diseases or conditions. A clinical registry is primarily used to measure quality of care, aiming to improve the quality by providing medical professionals and patients insights in the (patient reported) clinical outcomes of certain care paths. In addition, data can be used for additional purposes such as research or policy making.
What are clinical registries used for?
- Evaluating treatment outcomes and quality of care
- Identifying best practices and benchmarks for healthcare providers
- Supporting clinical research and public health initiatives
- Collecting real-world evidence for regulatory submissions and policy-making
Who can start a clinical registry?
Initiators of clinical registries may differ by country. Typically, clinical registries can be initiated by:
- Healthcare institutions (hospitals, clinics)
- Research organisations or academic institutions
- Professional medical societies or associations
- Government health agencies or regulatory bodies
What data points are typically collected for a clinical registry?
The content of a clinical registry is usually defined by medical professionals that initiate the registry. Key question here is which data points are needed to properly measure quality of care for the type of healthcare in scope of the registry. Data points collected for a clinical registry may include:
- Patient demographics (age, gender, ethnicity)
- Clinical data (diagnosis, treatment plans, interventions)
- Patient-reported outcomes (quality of life metrics, satisfaction)
- Follow-up data (complications, readmissions, survival rates)
- Healthcare resource utilisation (HCRU: costs, length of stay)
What does the output of a clinical registry look like?
The output of a clinical registry are insights in quality outcomes of the care path in scope. Which type of insights are created depends on the type of care in scope, and can be defined by the initiators of the registry. This output is made available to, amongst others, medical professionals and patients in reports, dashboards, and statistics that display:
- Patient demographics and cohort characteristics
- Treatment outcomes and effectiveness
- Comparisons against benchmarks or performance indicators
- VisualiSations such as graphs and tables for ease of interpretation
What rules and regulations must be considered when starting a clinical registry?
When establishing a clinical registry, several regulations must be considered, which can differ by country. In Europe, key regulations include:
- GDPR (General Data Protection Regulation): Ensures the protection of personal data and privacy for individuals within the EU, requiring explicit consent from patients for data collection.
- Local privacy and security laws: Each country may have specific laws governing health data management, data sharing, and patient consent.
- Ethics committee approval: Depending on the country, an ethical review board may need to approve the registry to ensure compliance with ethical standards.
What do you mean by 'ready-to-go-platform'?
The platform we have created for data collection, data validation, data analysis and sharing back benchmark and analytical data can be easily adjusted for any clinical registry and is essentially a plug-and-play solution.
How does LOGEX support data collection?
LOGEX offers various solution to collect large amounts of data to fill the registries. Through our platform, participating hospitals can perform bulk (batch) uploads and/or use the (FHIR)API. Through these automated processes we aim to reduce the administrative burden at the providers. Additionally there is the option to upload data manually. Where possible, we seek for smart data collection by re-using already existing data sources such as financial reimbursement data.
Does LOGEX support hospital participation?
Generally speaking, hospital recruitment is a task of the registry owner. With our vast experience with clinical registries, we can provide best practices and support the process. Also, after agreeing to participate we support hospitals in onboarding and participation (e.g. in data collection).
For most of our customers, after agreement of the hospital, we are in the lead in the contracting process as part of our user support activities. However, depending on the registry owner’s preferences we can flexibly define the scope of our service.
How are the outcomes reported to the registry owner?
We determine - in collaboration with the registry owner -what information and which analyses should be made available in the registry. These are reported in a customisable dashboard, called Codman. Additional data and analysis requests are always an option. LOGEX offers supports of a specialised Analytics team, that can support the registry owner in best defining the set-up of the registry (e.g. calculation rules and/or case-mix corrections).
Depending on the registry owners needs, various ways of data distribution can be organised. For example, we can discuss methodologies to create exports for scientific usage.
How are the outcomes reported to participating hospitals?
Our Codman dashboard can be configured based on the wishes of the registry owner. In these dashboards hospitals get insight in their own registry outcomes (in trends over time), can compare their outcomes against benchmarks and deep-dive on the data in various ways (e.g. patient overviews). Alternatively, they can opt to use the standard dashboards, which shows the most requested benchmarks and analyses.
Also, hospitals can be provided with their own (analyzed) data by requesting data automatically in the Data Request Application. Depending on the registry owner's needs, various ways of data distribution can be organised.
Benefit from our Healthcare Intelligence Suite
Go beyond Clinical Registries with Patient Engagement and Financial Analytics.

Patient Engagement
Optimise digital patient engagement activities while collecting valuable data.

Financial Analytics
Controlling costs and optimising operations through data-driven decision-making.
Secure, compliant and trusted.
Healthcare organisations worldwide trust LOGEX.
Improving perioperative care with a nationwide quality registry
An important aspect of this quality registry is providing a safe learning environment for clinicians. Data in this registry is meant to improve patient care and not for managers and insurance companies. A dashboard will be provided for the individual health care provider to compare their data to other groups but not to other individuals. We hope to improve patient care while providing a safe learning environment for healthcare providers.




Learn how to leverage data
to make better decisions.
Read our latest publications about how we support data-driven decision-making.

What does LOGEX do in the field of financial analytics?

My B-Pathway’ Innovative Online Portal for Breast Cancer Patients by LOGEX Patient Engagement

How can real-world evidence be used to solve unwarranted variation in drug up take and clinical guidelines?
Get clarity and control to make better decisions.
Do you have any questions about harnessing data-driven insights for better decision-making? Allow our experts, such as Sarah, to lead the way.
We will reply within two business days.