- Solutions
- Real World Evidence
- IMID Observatory
Gain unique international insights
Actionable insights into drug use and effectiveness
Gain insights into the uptake of a specific drug, the use of a specific drug in patient pathways, and the effectiveness of the drug in a standardised manner.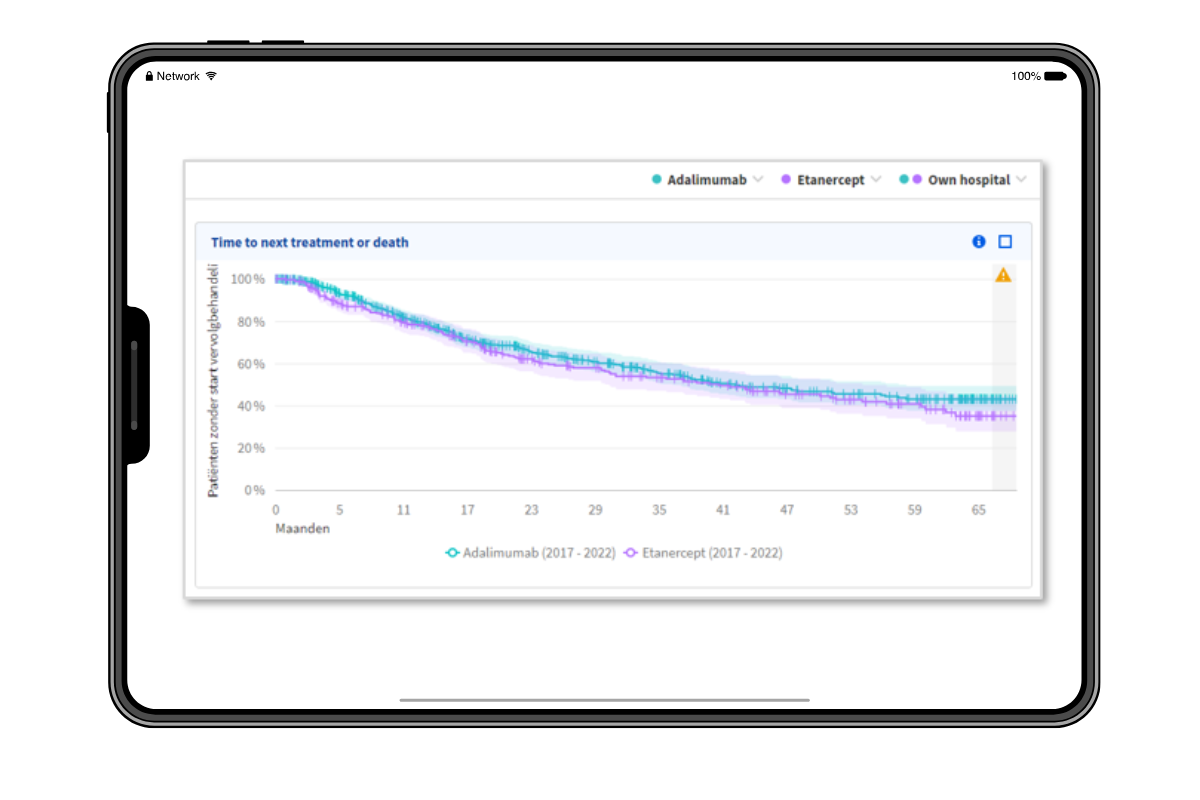
Hospitals participate burden free
We run treatment improvement projects that are easy to join. They are, by design, completely compliant with all legal requirements around ethics, data security, and privacy.
Unlocking the value of RWD
By enabling the use of real-world data that reflect the actual population treated, it is possible to assess treatment effects. This makes timely decision-making easy and evidence-driven.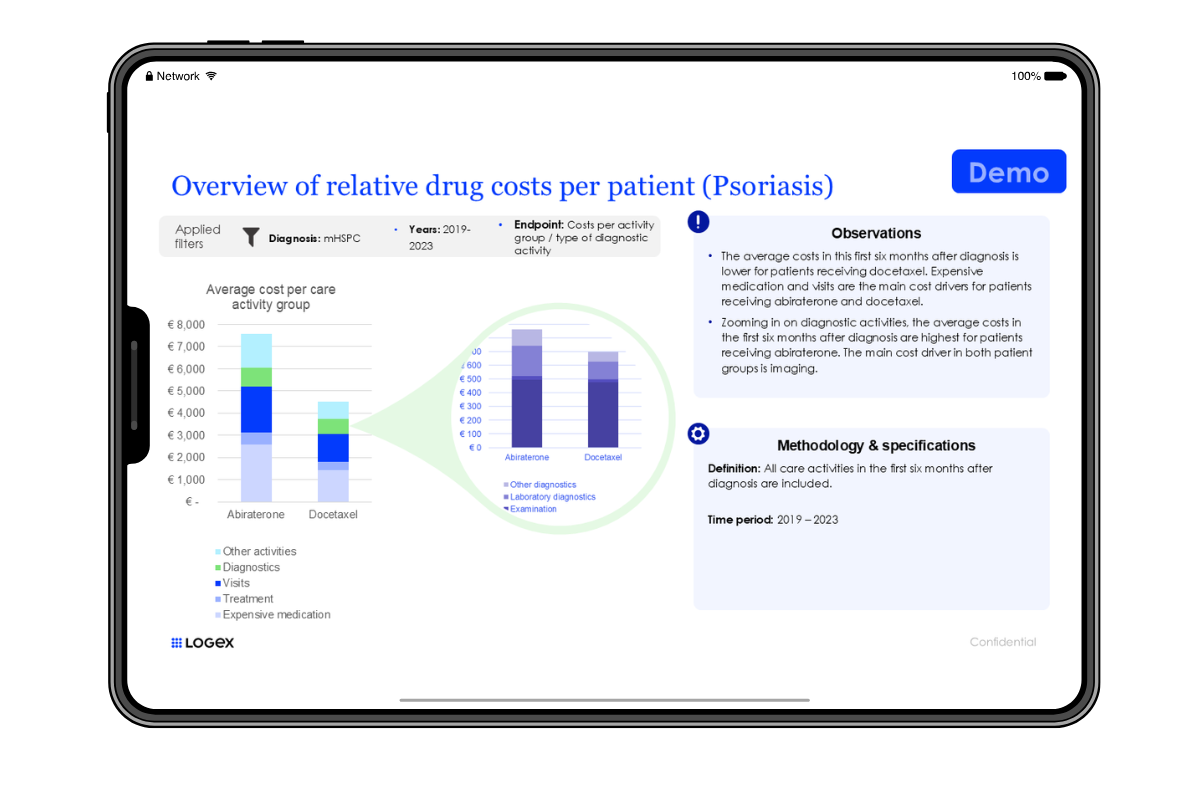
What makes our Observatory approach unique?

Data granularity
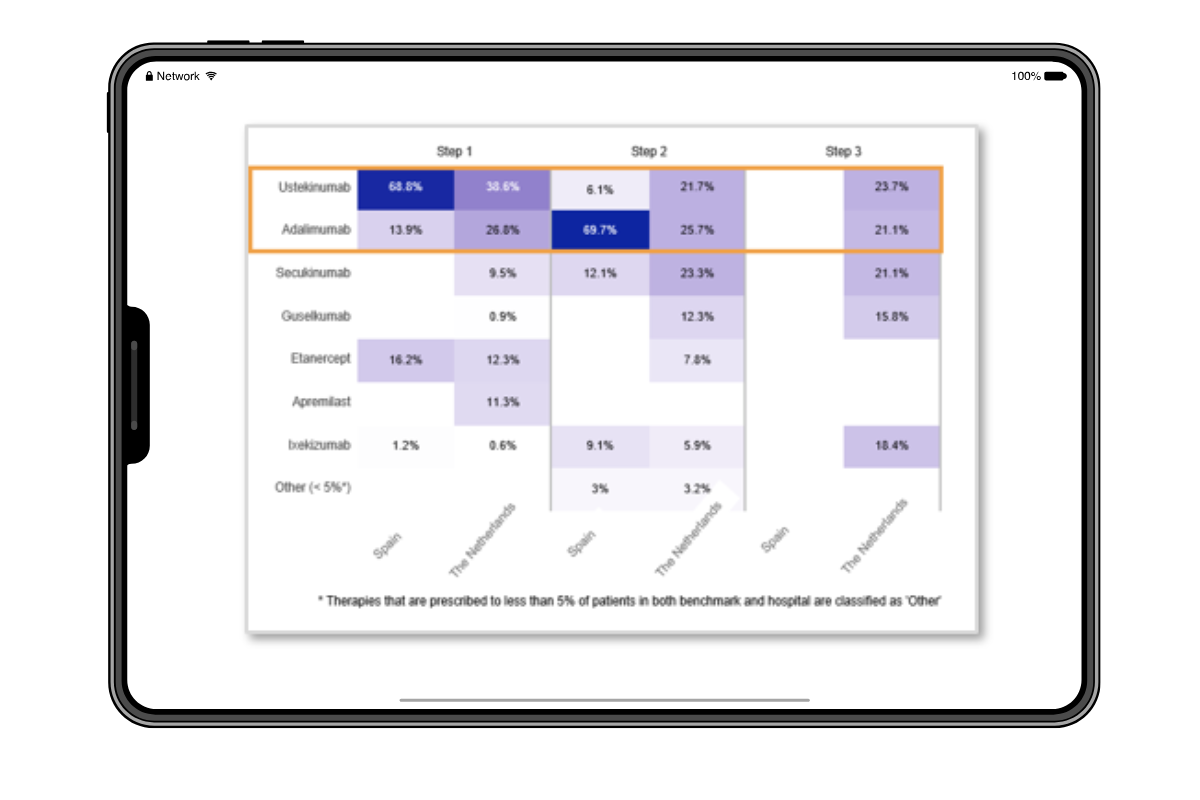
The level of detail LOGEX has access to is unique, allowing exceptional visibility into patient pathways. E.g.:
- Patient pathway mapping
- Lines of treatment
- Combinations of drugs and indication split
- Filter on specific patient groups

Data access & recency

Longitudinal insights

Scalability
The observatory setup allows for quick expansion into related disease areas under the same contracts.
With our extensive network of hospitals across Europe, expanding into other countries is highly feasible.

Experience
LOGEX has over 10 years of experience working with 700+ healthcare providers across the EU.
We are experts in extracting, delivering, validating and analysing healthcare datasets in close collaboration with HCPs.

Security by design
NEN7510 and ISO27001 certified, full compliance with GDPR.
FG and security officers, authorisation levels, two-factor authentication, informed / explicit consent.
Frequently Asked Questions
What is the legal basis for processing data in the Observatories?
The legal basis for processing data in our projects is provided by GDPR article 9.2(h), which covers projects designed to improve "the management of health or social care systems and services."
Who is responsible for data management, and how is it structured?
The hospital is the data controller, while LOGEX acts as the data processor, ensuring data is handled according to the legal and ethical guidelines.
Are there any specific requirements or ethical approvals needed for the Observatories?
Ethical approval might be necessary in specific cases and is dependent on local guidance and regulations. The focus of the observatory is to improve healthcare and outcomes for participating hospitals, which means ethical approval is not required in certain countries or specific situations.
How is hospital data handled and are there any restrictions on sharing it?
Hospitals remain the owners of their data at all times. Only anonymous population data may be shared with third parties, and LOGEX strictly follows local regulations regarding cross-border data transfers.
Get more out of our Healthcare Intelligence
Go beyond Real-World Data with Patient Engagement and Financial Analytics.

Patient Engagement
Optimise digital patient engagement activities while collecting valuable data.

Financial Analytics
Controlling costs and optimising operations through data-driven decision-making.
Secure, compliant and trusted.
Healthcare organisations worldwide trust LOGEX.
Breaking Barriers in International Psoriasis Research Through Data Standardisation
Discover how LOGEX is transforming international psoriasis research through innovative data standardisation, enabling faster, more reliable insights across Europe.
Actionable insights into innovative medicines and their costs
Report: "On the Horizon".
March 2024 edition.

Learn how to leverage data
to make better decisions.
Read our latest publications about how we support data-driven decision-making.

Solidifying Oncology Drug Effectiveness After Approval with Real-World Evidence

How to establish innovative medicines pricing to improve patient outcomes and reward value instead of volume
-1.jpg?width=1732&height=1155&name=Stocksy_txp3c7f9c9dMmT300_Medium_2944384-edit%20(1)-1.jpg)
How can RWE be used to solve unwarranted variation in drug uptake and clinical guidelines?
Get clarity and control to make better decisions.
Do you have any questions about harnessing data-driven insights for better decision-making? Allow our experts, such as Sarah, to lead the way.
We will reply within two business days.


%20(7).png?width=1200&height=800&name=Untitled%20(600%20x%20400%20px)%20(7).png)